One of the most misused and misunderstood sensory terms in evaluating coffee’s taste is acidity. It is a quantitative term that refers to the relative strength of the acids present in the coffee. And though the terms acidy and acidity are related, the two are not interchangeable.
Acid is a chemical compound that contains hydrogen atoms that are capable of giving off protons (hydrogen ions, if you’re curious). This condition can be measured quantitatively. A food technologist would tell you virtually all beverages are acidic. There relative strengths, for instance, can be measured by a pH number, which is the quantitative measure of the presence of free hydrogen ions.
Coffee contains a wide variety of acids, most of which are found in other agricultural products. These acids include amino acids (asparagine, glutamic and leucine); phenolic acids (caffeic, chlorogenic and quinic); and aliphatic acids (acetic, lactic, citric, malic, fumaric, oxalic, phosphoric and tartaric). From a taste perspective, higher levels of amino acids lead to a sweet sensation, higher levels of phenolic acids lead to a bitter sensation, and higher levels of aliphatic acids lead to a sour sensation.
Phenolic Acids
The phenolic acid group accounts for the largest percentage (by volume) of the acids found in coffee. By far, the largest component of this group is chlorogenic acid. Chlorogenic acid (which includes three sub-groups) plays an important role in determining the flavor of freshly brewed coffee. It is fairly unstable and decomposes into caffeic and quinic acid as the coffee sits in the pot, particularly at temperatures above 185 degrees or below 175 degrees Fahrenheit. Once split apart, the quinic acid has a noticeably bitter taste, and the caffeic acid is recognizably sour. Together, this combination of bitter and sour create the acerbic taste and smell of old coffee.
Aliphatic Acids
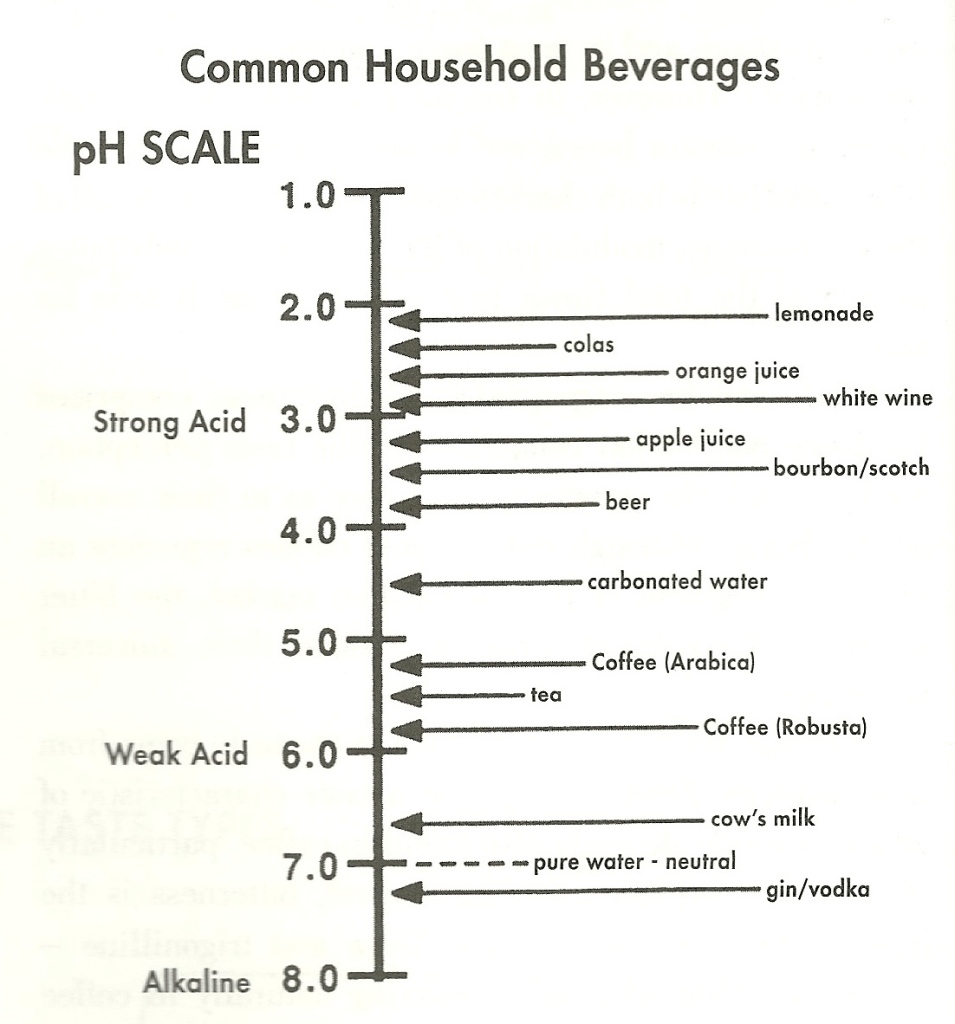
While not the greatest by volume, aliphatic acids tend to produce the greatest quantity of hydrogen ions (which can be measured on the pH scale). The increased concentration of hydrogen ions results in sourness. The order of the intensity of this group of acids is generally considered as: tartaric, citric, malic, lactic and acetic. The increased concentration of aliphatic acids can also impact the perception of other basic tastes, particularly sweet.
Each individual acid in this category has its own unique flavor, such as the lemony flavor of citric acid, the buttery flavor of lactic acid and the apple-like flavor of malic acid. These attributes are often more perceptible as odors rather than tastes. Acetic acid is the exception in this group. When it is present in brewed coffee, it’s typically the result of the fermentation process in washed coffee. If too much acetic acid is formed, the green beans develop a characteristic fruity smell that is a precursor to the highly objectionable fermented taste it produces in brewed coffee.
In a general sense, the presence of aliphatic acids give coffee a brightness and zest to its flavor. It is the underlying reason why coffees with a high acidity (low pH value of 4.8 to 5.1) often are sold at premium prices.
